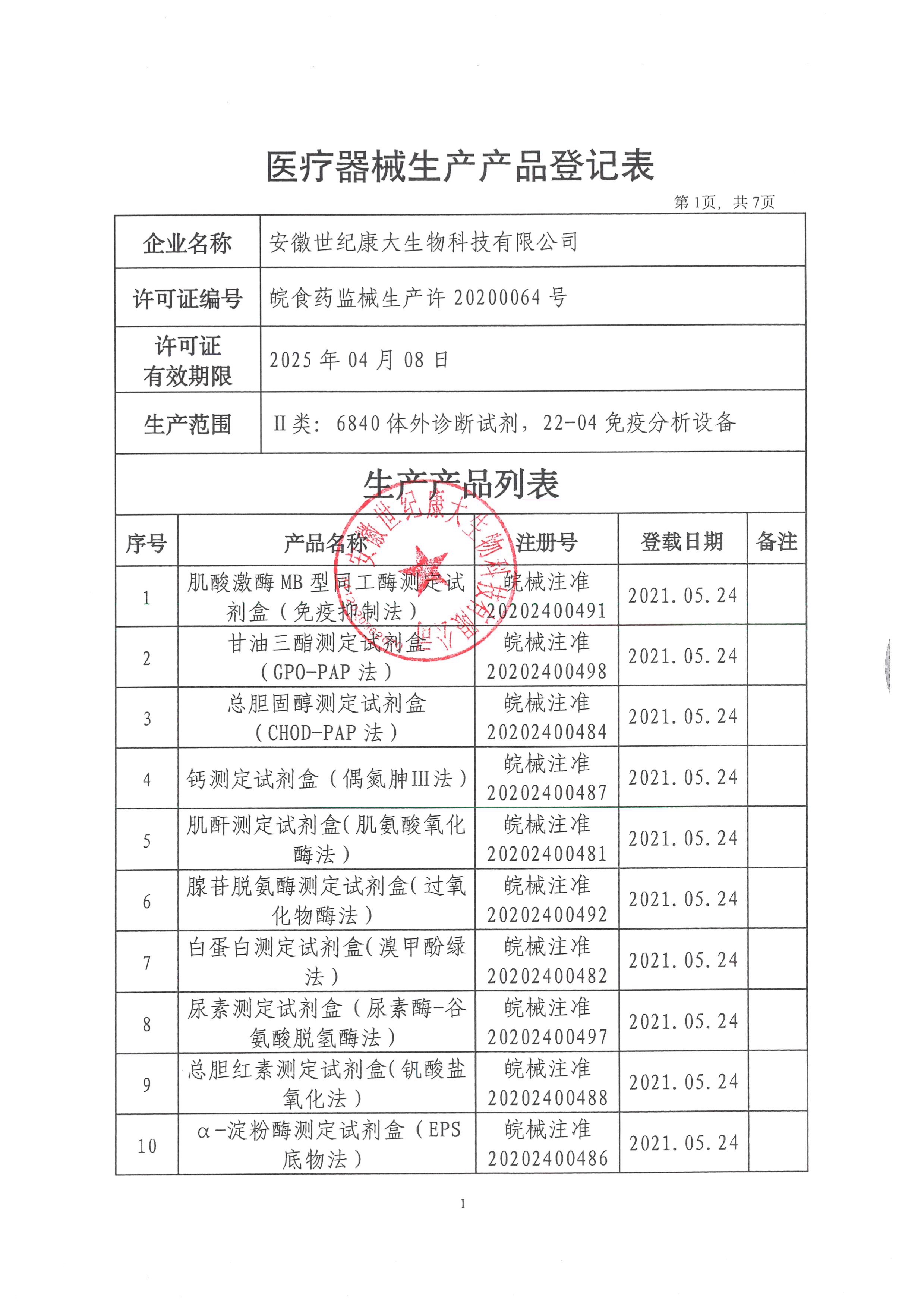
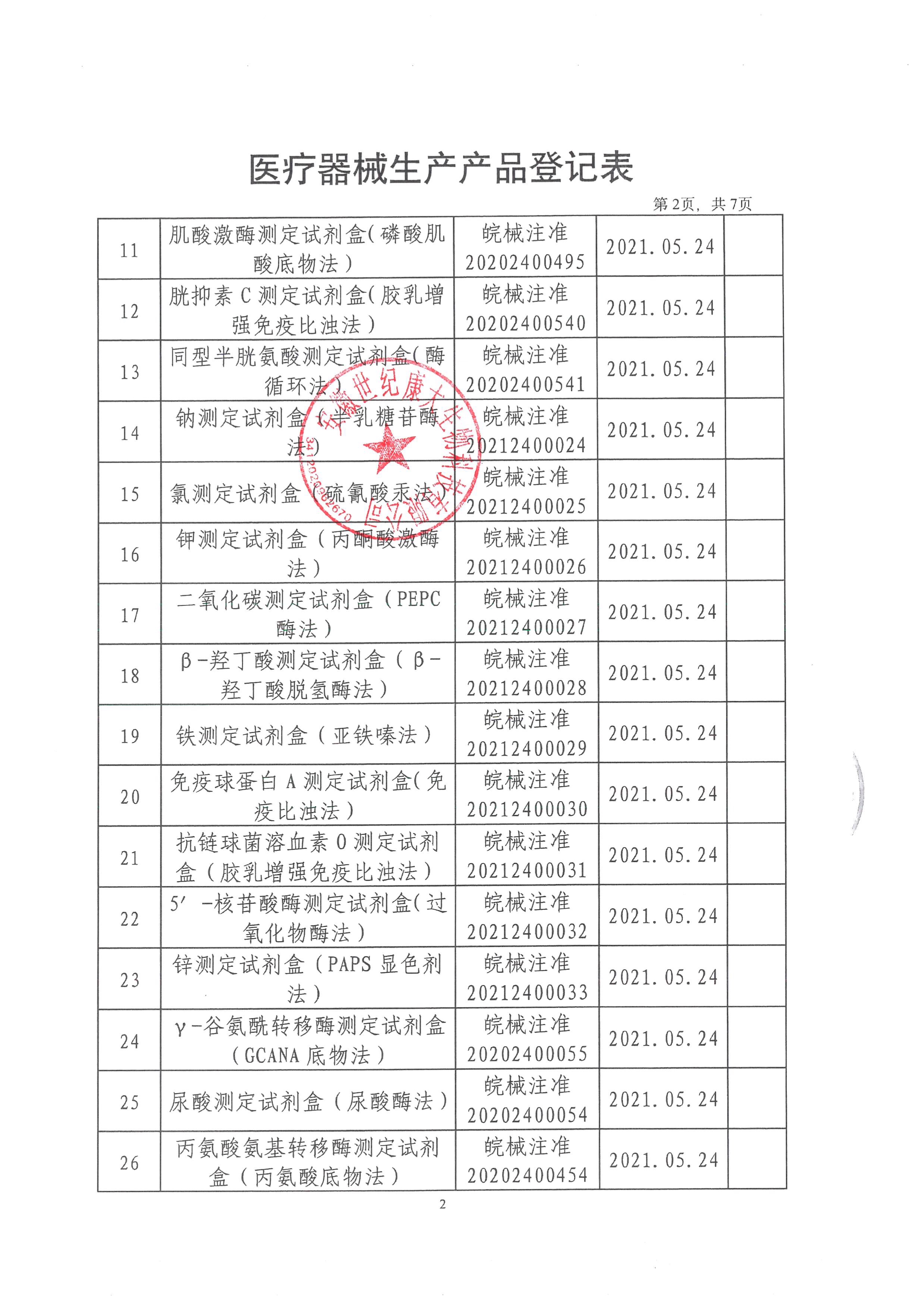
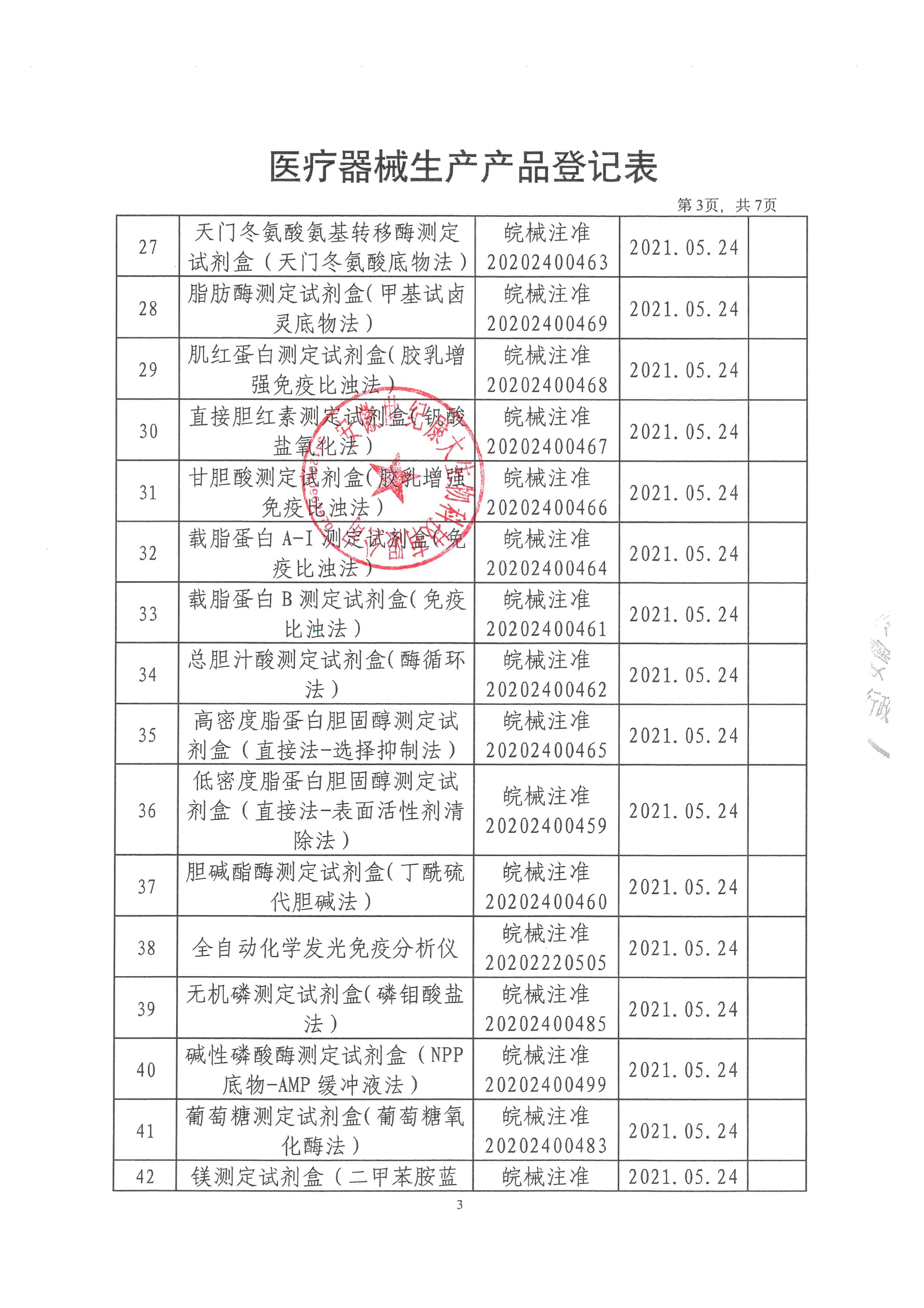
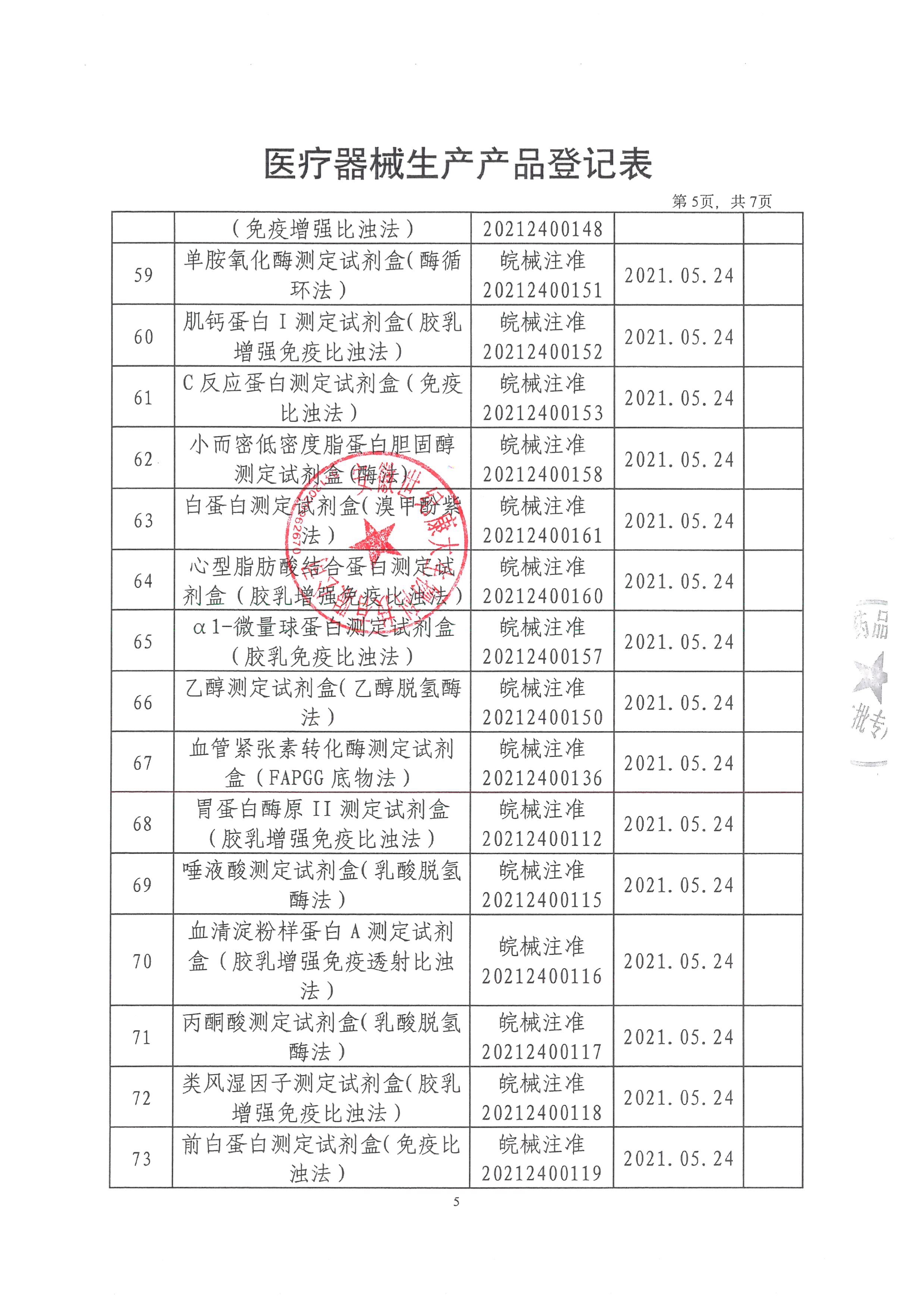
Anhui Century Kangda Biotechnology Co., Ltd., founded in 2018, is a key investment attraction project in Fuyang City and the first company in Fuyang to do in vitro diagnostic reagent products and instruments. It is located in the high-end medical equipment industrial park of Fuyang Economic Development Zone, covering an area of 3600 square meters, including 309 square meters of 100000 and 10000 level clean workshops, 97 square meters of quality inspection area, and 85 square meters of cold storage area. The main construction contents include: finished product warehouse, intermediate product warehouse, sample retention warehouse, preparation room, weighing room, packaging room, subpackaging room, fan room, water preparation room, quality department, etc. Main business: production and sales of in vitro diagnostic biochemical reagents. At present, Anhui Century Kangda has obtained 130 Class II medical device registration certificates of in vitro diagnostic reagents and two Class II instrument registration certificates. At present, the products produced by Anhui Century Kangda basically cover the immunoluminescence and large biochemical projects in the laboratory of Class III A hospitals. The company is equipped with a professional after-sales service team, and each engineer has received professional training from the manufacturer. At the same time, the company implements the "first question responsibility system" for customer services to truly ensure that every request of each customer is answered at the first time. Anhui Century Kangda, which continues to pay attention to human health, is committed to promoting national products in the field of in vitro diagnosis. Relying on the value concept of "focusing on medical treatment, innovation as the soul, smiling service, and win-win cooperation", relying on clinical biochemical detection reagents, we focus on the research of immunoluminescence products, integrate and optimize various resources, and become a first-class intensive service provider in the field of in vitro diagnosis in China.
After more than three years of development, the company has invested nearly 200 million yuan. At present, the company has approved 130 Class II reagent certificates and two Class II instrument certificates. We will strive to be the manufacturer with the largest number of medical device certificates in China within three years. After fully expanding sales, the annual sales volume can reach 200 million. The company's customers cover more than 800 users and medical institutions across the country. The company has become one of the most potential enterprises in the domestic IVD industry. The company will seize the opportunity of the vigorous development of the domestic IVD industry, develop and improve itself, and provide customers with first-class products and after-sales services.